ELIMINATING M. HYO
Mycoplasma hyopneumoniae, or M. hyo, is a host-specific organism that only infects pigs. It is the primary agent of enzootic pneumonia, a chronic respiratory disease that can act as a gateway letting mixed respiratory infections from secondary pathogens into the pig’s system.
INFECTION PATHWAY
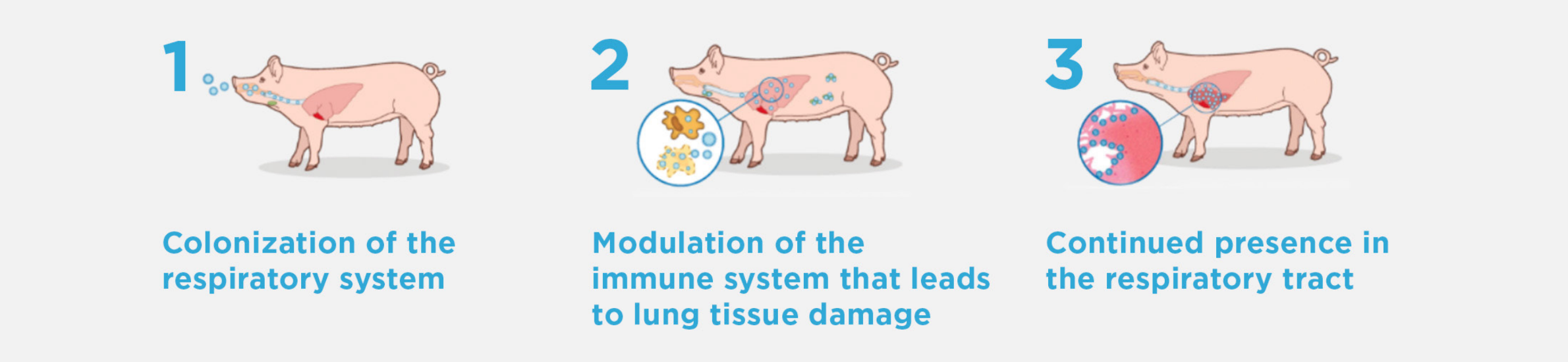
M. hyo is slow growing between 1 & 2, may be weeks to months; that is why piglets can be exposed preweaning but not show clinical signs until finishing phase.
Pigs in any age group can be infected by M. hyo, but infection dynamics do vary from farm to farm and are dependent on factors such as handling, type of housing, and production system. While transmission between herds occurs mainly through the introduction of infected animals, within a herd you can distinguish between vertical and horizontal transmission:
> Vertical transmission is from the sow to the offspring, typically nose-to-nose contact. Although the risk is lower than in younger sows, older sows may still transmit M. hyo to their piglets.
> Horizontal transmission is between pigs of the same or different pens by direct or indirect contact.
Economic losses from M. hyo-related swine respiratory disease (SRD) show up in reduced performance gains, uneven growth, more days needed to reach slaughter weight, treatment and control costs, and even increased mortality as a result of complicated infections.1
BATTLING THE ENEMY
Because there is evidence of little genetic variability of M. hyo within swine production flows, this generally enables a successful herd-wide elimination program. Of all the elimination pathways, exposure and closure, along with medication, is the most prevalent and has proven the most successful long-term tactic. Though this is an economic investment, elimination has shown a good success rate when protocols are implemented correctly. Even if the breeding herd remains negative for only one year, the investment is still worthwhile due to the economic benefits downstream.2





MONITORING THE CLOSE
During herd closure, focus on the progression of M. hyo through the breeding animals. Observe clinical signs to alleviate symptoms with strategic medication programs as directed by your veterinarian. Other management practices can also be implemented at this time to minimize transmission of M. hyo from breeding herd to nursing piglets10,11 and assist in reducing disease in later grow-finish during the elimination.
A combination of biosecurity and persistence is necessary to see this pathogen eliminated from the breeding herd. Success is defined when pigs emerging from the naive replacement gilts reach late grow-finish and pre-market stage (about six months after completion of herd closure) and M. hyo clinical signs are lacking, along with minimal medication interventions.
Talk to your herd veterinarian and our Elanco Technical Consultant on medication program options.
The labels contain complete use information, including cautions and warnings. Always read, understand and follow the label and use directions.
PULMOTIL® AC IMPORTANT SAFETY INFORMATION:
Before using this product, it is important to read the entire product insert, including the boxed human warning.
WARNING: Exposure to tilmicosin in humans has been associated with chest pain, increased heart rate, dizziness, headache, and nausea. Death has been reported following ingestion or injection of tilmicosin. Avoid direct skin and eye contact. In case of human exposure, call 1-800-722-0987 and consult a physician immediately.
• Wear overalls, impervious gloves and eye protection when mixing and handling the product. Wash hands after handling the product. Wash affected parts if skin contact occurs. If accidental eye contact occurs, immediately rinse thoroughly with water.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
• For use only in swine. Not for injection. Injection of tilmicosin has been shown to be fatal in swine and non-human primates, and may be fatal in horses and goats.
• Swine intended for human consumption must not be slaughtered within 7 days of treatment.
• Always treat the fewest number of animals necessary to control a respiratory disease outbreak. Prescriptions shall not be refilled.
• Concurrent use of Pulmotil AC and another macrolide by any route, or use of another macrolide immediately following this use of Pulmotil AC is not advised.
• Ensure that pigs have continuous access to medicated water during the treatment period. Monitor pigs for signs of water refusal and dehydration while being treated.
BAYTRIL® IMPORTANT SAFETY INFORMATION:
CAUTION: Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian. Federal (U.S.A.) law prohibits the extra-label use of this drug in food-producing animals. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.
• Not for use in humans. Keep out of reach of children.
• Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes.
• In case of dermal contact, wash skin with soap and water. Consult a physician if irritation persists following ocular or dermal exposures.
• Individuals with a history of hypersensitivity to quinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight.
INCREXXA™ IMPORTANT SAFETY INFORMATION:
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
WARNINGS: FOR USE IN ANIMALS ONLY. NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. NOT FOR USE IN CHICKENS OR TURKEYS.
• Swine intended for human consumption must not be slaughtered within 5 days from the last treatment.
• The effects of Increxxa on porcine reproductive performance, pregnancy, and lactation have not been determined.
• Intramuscular injection can cause a transient local tissue reaction that may result in trim loss of edible tissue at slaughter.
• Store below 25°C (77°F), with excursions up to 40°C (104°F).
• 100 mL: Use within 2 months of first puncture and puncture a maximum of 67 times. If more than 67 punctures are anticipated, the use of multi-dosing equipment is recommended. When using a draw-off spike or needle with bore diameter larger than 16 gauge, discard any product remaining in the vial immediately after use.
• 250 mL and 500 mL: Use within 2 months of first puncture and puncture a maximum of 100 times. If more than 100 punctures are anticipated, the use of multi-dosing equipment is recommended. When using a draw-off spike or needle with bore diameter larger than 16 gauge, discard any product remaining in the vial immediately after use.
