Pulmotil® AC (tilmicosin phosphate) and Pulmotil® Premix (tilmicosin)

Pulmotil® AC (tilmicosin phosphate) and Pulmotil® Premix (tilmicosin)
Pulmotil® AC: For the control of swine respiratory disease (SRD) associated with Pasteurella multocida (PM) and Glassaerella (Haemophilus) parasuis. For the control of SRD associated with Mycoplasma hyopneumoniae (M. hyo) in the presence of PRRS in groups of swine in buildings where a respiratory disease outbreak is diagnosed. Pulmotil® Premix: For the control of SRD associated with Actinobacillus pleuropneumoniae (APP) and PM.
Package Size:
Pulmotil Premix: 10 kg, Pulmotil AC: 960 mL
Flexible SRD Control
- Quick.
- Flexible.
- Effective.
- Easy.
Tilmicosin Mode of Action
Disease enters the system and is treated
Pulmotil goes to work and reinforces natural defense system
Pulmotil kills bacteria
Estimated Loss From Respiratory Disease1

Pulmotil AC Data
Lung Lesion Percentage Results Following 5-Day Treatment Period2
Effectiveness in PRRSv and M. Hyo co-infection

Pulmotil Premix Data
Impact of Pulmotil Premix When Fed in Lactation Ration3
Effectiveness to control SRD associated with APP and P. multocida on a 1,300-sow multi-site farrow-to-finish operation with a history of SRD.

Pigs per Litter
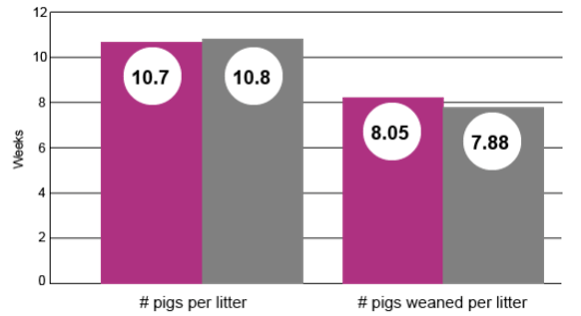
No significant difference in pigs per litter or pre-weaning mortality. (P = 0.6654 and P = 0.6335, respectively.)
Weight at Weaning (avg, lbs)
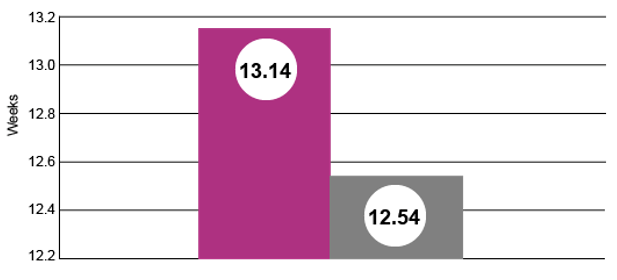
Pigs from sows fed Pulmotil in lactation to control SRD weighed greater than a half-pound more at weaning than pigs from sows with no antibiotics in lactation. (P < 0.0001)
Nursery Mortality (%)
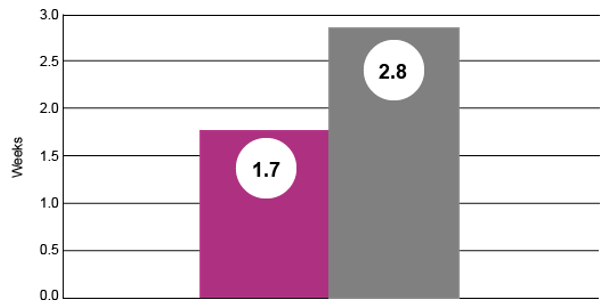
Nursery mortality was 39% lower in pigs weaned from sow treated with Pulmotil in lactation to control SRD. (P = 0.0177)
Weight at Six Weeks Post-weaning (% pigs ≤ 25 lbs)
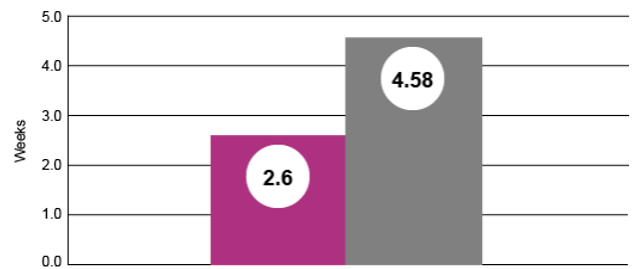
At six weeks post-weaning, nearly 2% fewer pigs from sows fed Pulmotil in lactation to control SRD were ≤ 25 lbs. (P = 0.0134)
Flexible SRD Control, No Injections
- Pulmotil sets a foundation for health management by controlling SRD associated with APP and PM. Feeding Pulmotil for the duration of lactation (21 days) to control respiratory disease in lactating sows decreases mortality in the farrowing and nursery phase.3
- Attached lungs, caused by respiratory disease lesions in the nursery, remain even after the pig recovers — leading to performance losses and packer penalties.4*

Pulmotil Premix Product Label
Read the full label for important use and safety information.
Get Full Value from start to finish
Our portfolio offers solutions that manage disease challenges and mitigate mortality to optimize health.
Intestinal Integrity
Elanco offers feed, water-soluble and injectable intestinal integrity solutions.
Respiratory Health
Managing both enteric and respiratory health challenges are critical to your herd's success.
The labels contain complete use information, including cautions and warnings. Always read, follow and understand the label and use directions.
Pulmotil® AC
IMPORTANT SAFETY INFORMATION:
Before using this product, it is important to read the entire product insert, including the boxed human warning.
WARNING: Exposure to tilmicosin in humans has been associated with chest pain, increased heart rate, dizziness, headache, and nausea. Death has been reported following ingestion or injection of tilmicosin. Avoid direct skin and eye contact. In case of human exposure, call 1-800-722-0987 and consult a physician immediately.
- Wear overalls, impervious gloves and eye protection when mixing and handling the product. Wash hands after handling the product. Wash affected parts if skin contact occurs. If accidental eye contact occurs, immediately rinse thoroughly with water.
INDICATIONS:
- For the control of swine respiratory disease associated with Pasteurella multocida and Haemophilus parasuis in groups of swine in buildings where a respiratory disease outbreak is diagnosed.
- For the control of swine respiratory disease associated with Mycoplasma hyopneumoniae in the presence of porcine reproductive and respiratory syndrome virus (PRRSv) in groups of swine in buildings where a respiratory disease outbreak is diagnosed.
DOSAGE AND ADMINISTRATION:
- Must be diluted before administration to animals.
- Include in the drinking water to provide a concentration of 200 mg tilmicosin per liter (200 ppm).
- One 960 mL bottle is sufficient to medicate 1200 liters (320 gallons) of drinking water for pigs.
- The medicated water should be administered for (5) five consecutive days.
- Use within 24 hours of mixing with water.
- Do not use rusty containers for medicated water as they may affect product integrity.
- When using a water medicating pump with a 1:128 inclusion rate, add 1 bottle (960 mL) of Pulmotil AC per 2.5 gallons of stock solution.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
- For use only in swine. Not for injection. Injection of tilmicosin has been shown to be fatal in swine and non-human primates, and may be fatal in horses and goats.
- Swine intended for human consumption must not be slaughtered within 7 days of treatment.
- Always treat the fewest number of animals necessary to control a respiratory disease outbreak. Prescriptions shall not be refilled.
- Concurrent use of Pulmotil AC and another macrolide by any route, or use of another macrolide immediately following this use of Pulmotil AC is not advised.
- Ensure that pigs have continuous access to medicated water during the treatment period. Monitor pigs for signs of water refusal and dehydration while being treated.
Pulmotil® Premix
INDICATIONS:
- For the control of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae and Pasteurella multocida.
FEEDING DIRECTIONS:
- Tilmicosin is to be fed continuously at 181 grams to 363 per ton (200 ppm to 400 ppm) of Type C medicated feed as the sole ration for a 21 day period, beginning approximately 7 days before an anticipated disease outbreak.
IMPORTANT SAFETY INFORMATION:
CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian.
- Do not allow horses or other equines access to feeds containing tilmicosin.
- Feed containing tilmicosin shall not be fed to pigs for more than 21 days during each stage of production without ceasing administration for reevaluation of antimicrobial use by a licensed veterinarian before re-initiating a further course of therapy with an appropriate antimicrobial.
- Veterinary Feed Directive (VFD) expiration date for swine must not exceed 90 days from the time of issuance. VFDs for tilmicosin phosphate shall not be refilled.
WARNINGS: Swine intended for human consumption must not be slaughtered within 7 days of the last treatment of this drug product.

