Pradalexᵀᴹ (pradofloxacin injection)

Pradalexᵀᴹ (pradofloxacin injection)
For the treatment of swine respiratory disease (SRD) associated with Bordetella bronchiseptica, Glaesserella (Haemophilus) parasuis, Pasteurella multocida, Streptococcus suis, and Mycoplasma hyopneumoniae (M. hyo).
Bottle Size

100 mL; 250 mL
SRD Powerhouse
- Fast.
- Effective.
- Convenient.
MODE OF ACTION
As a new, third-generation fluoroquinolone, Pradofloxacin has a unique structure that differs from any other molecule in the class. Pradalex’s mode of action has an equal affinity to both DNA gyrase and topoisomerase IV, resulting in a dual targeting effect and increased potency relative to other fluoroquinolones.
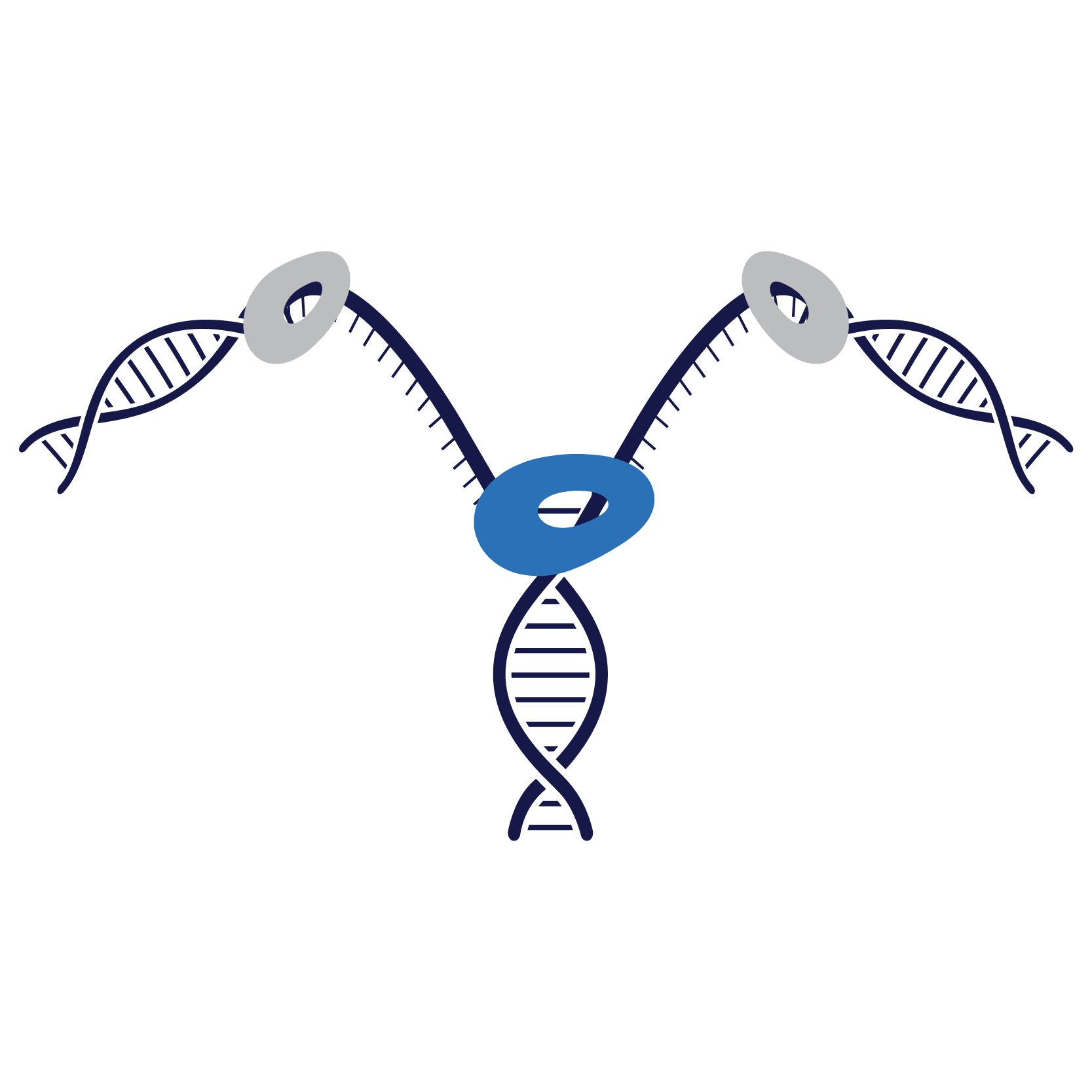
In bacterial replication, the enzyme DNA gyrase unfolds the bacterial DNA for replication by DNA polymerase.
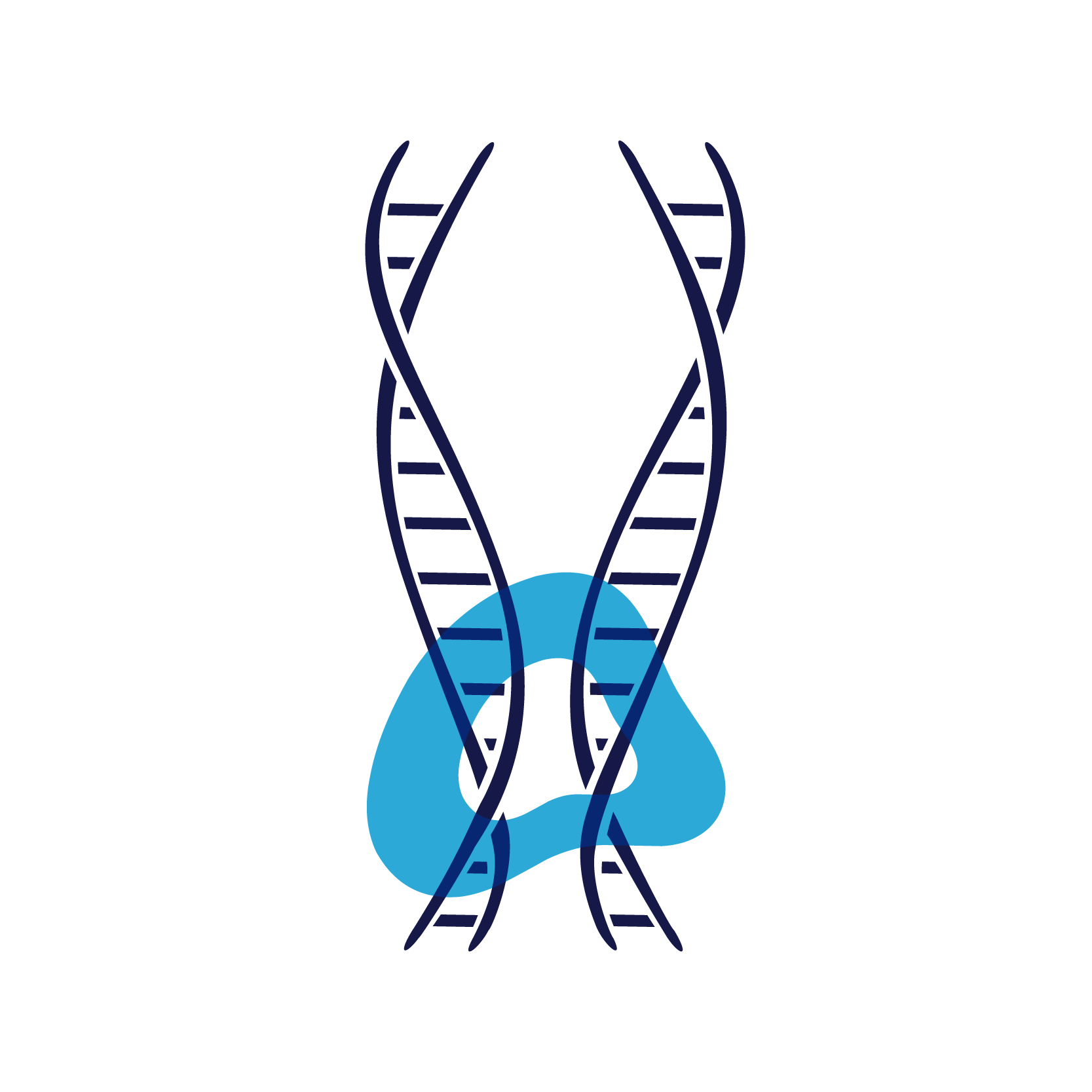
Afterward, the enzyme topoisomerase IV separates the identical DNA copies into sister cells.
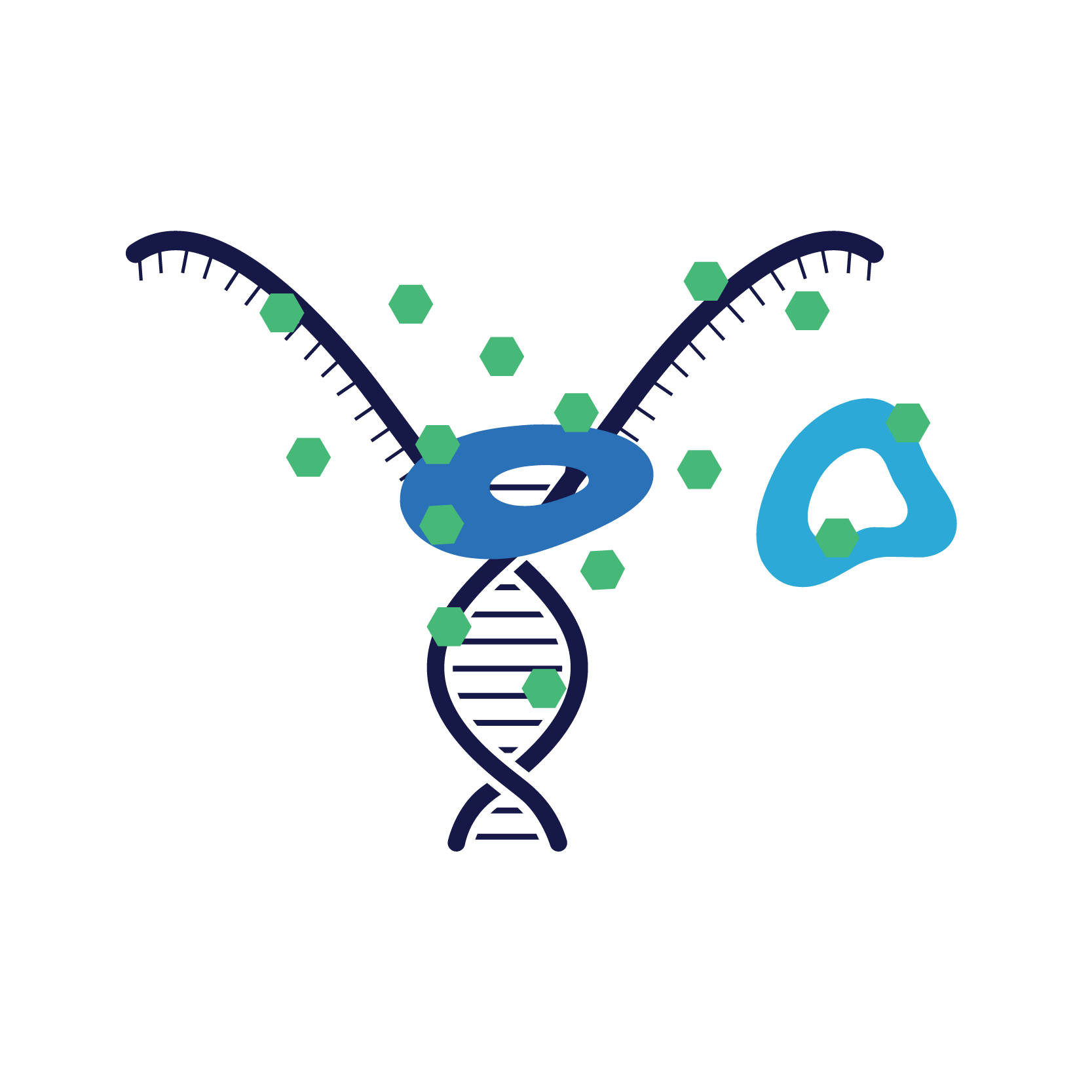
Pradalex works by simultaneously binding and inactivating both DNA gyrase and topoisomerase IV inhibiting bacterial replication.
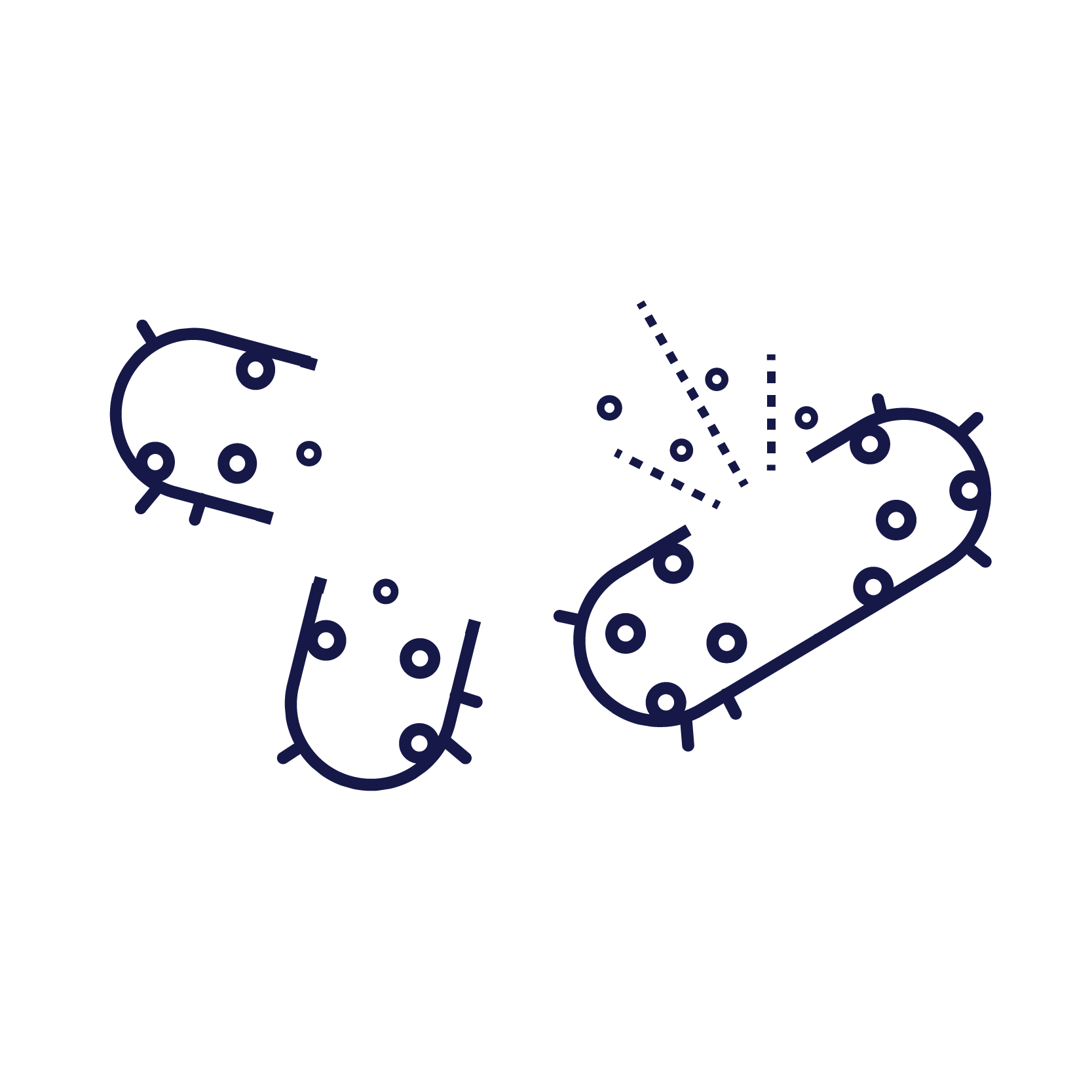
This causes the chromosome to fragment and results in rapid cell death.

Dosage and Administration
Administer once as an intramuscular injection in the neck at a dosage of 7.5 mg/kg (1.7 mL/100 lbs) body weight. Do not inject more than 5 mL (290 lbs) per intramuscular injection site.
Industry-leading 2-day withdrawal period.
Single-dose, low-volume antibiotic with exceptional syringeability3
A Powerful SRD Punch
- Pradalex is an SRD antibiotic treatment that reaches twice the concentration in a third of the time1,2 — achieving peak serum activity within 45 minutes after injection.
- Featuring a unique molecular structure and mode of action, Pradalex simultaneously blocks two enzymes responsible for bacterial replication within the bacteria nucleus, leading to improved potency and broad-spectrum efficacy relative to other injectable antibiotics.
- Pradalex is a convenient, one-shot, low-volume antibiotic with a 2-day withdrawal period, offering SRD treatment protocol flexibility from nursery to finish.

Get Full Value from start to finish
Our portfolio offers solutions that manage disease challenges and mitigate mortality to optimize health.
Respiratory Health
Managing both enteric and respiratory health challenges are critical to your herd's success.
The label contains complete use information, including cautions and warnings. Always read, follow and understand the label and use directions
INDICATIONS:
Pradalex is indicated for the treatment of SRD associated with Bordetella bronchiseptica, Glaesserella (Haemophilus) parasuis, Pasteurella multocida, Streptococcus suis and Mycoplasma hyopneumoniae in weaned swine intended for slaughter (nursery, growing, and finishing swine, boars intended for slaughter, barrows, gilts intended for slaughter, and sows intended for slaughter). Not for use in swine intended for breeding (boars intended for breeding, replacement gilts and sows intended for breeding) and in nursing piglets.
DOSAGE AND ADMINISTRATION:
Swine: Administer once as an intramuscular injection in the neck at a dosage of 7.5 mg/kg (1.7 mL/100 lb) body weight. Do not inject more than 5 mL per intramuscular injection site.
IMPORTANT SAFETY INFORMATION:
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Not for use in humans. Keep out of reach of children. Avoid contact with eyes and skin. Individuals with a history of hypersensitivity to quinolones should avoid this product. Not for use in animals intended for breeding because the effects of Pradalex on swine reproductive performance, pregnancy and lactation have not been determined. Not for use in nursing piglets because safety and effectiveness have not been demonstrated. Quinolones should be used with caution in animals with known or suspected central nervous system (CNS) disorders. Mild to moderate inflammatory changes of the injection site may be seen in swine treated with Pradalex. See package insert for additional safety information.

