AN INNOVATIVE BRD AND SRD POWERHOUSE
INTRODUCING PRADALEXTM (pradofloxacin injection)
Bovine respiratory disease (BRD) and Swine respiratory disease (SRD) remain a major challenge, even as management practices and interventions continue to improve. PradalexTM brings a novel mode of action with a hard-hitting bactericidal efficacy against all relevant BRD and SRD pathogens.
SIGNATURE CHARACTERISTICS
Pradofloxacin is an innovative, third-generation fluoroquinolone
Blocks two enzymes responsible for bacterial replication with equal affinity in the same organism
Achieves peak activity in the lungs in 45 minutes in cattle
Convenient single-dose, low-volume injection
PRADALEX IS READY TO TAKE ON:
BRD BACTERIA

Histophilus somni
Mannheimia haemolytica
Mycoplasma bovis

Pasteurella multocida
SRD BACTERIA
Bordetella bronchiseptica

Glaesserella (Haemophilus) parasuis

Pasteurella multocida
Streptococcus suis
Mycoplasma hyopneumoniae
BRINGING YOU FAST* AND STRONG BACTERICIDAL ACTIVITY
PRADALEX IN ACTION
Fluoroquinolone antibiotics work by binding and inactivating DNA gyrase and topoisomerase IV. This inhibits bacterial replication and causes the chromosomes to fragment into pieces — resulting in cell death. Most fluoroquinolones act primarily on DNA gyrase but Pradalex does it differently.
Pradalex's dual targeting effect yields improved potency, faster bactericidal activity, and a broader spectrum of activity relative to other fluoroquinolone antibiotics.1
Pradalex is ready to go to work for your operation.
POWERFUL PUNCH, SMALL DOSE
Pradalex brings the benefits without compromising on delivery. This antibiotic is a one-shot, low-volume injection — with exceptional syringeability.2
Pradalex is rapidly absorbed, effectively reduces morbidity and mortality, and is cleared quickly with an industry-leading withdrawal period. This decreases the period where selection for resistant bacteria occurs, contributing to judicious antibiotic use.
Hard-hitting against bacteria. Easy on your production teams. Pradalex is designed to be your go to first treatment to fight BRD and SRD.
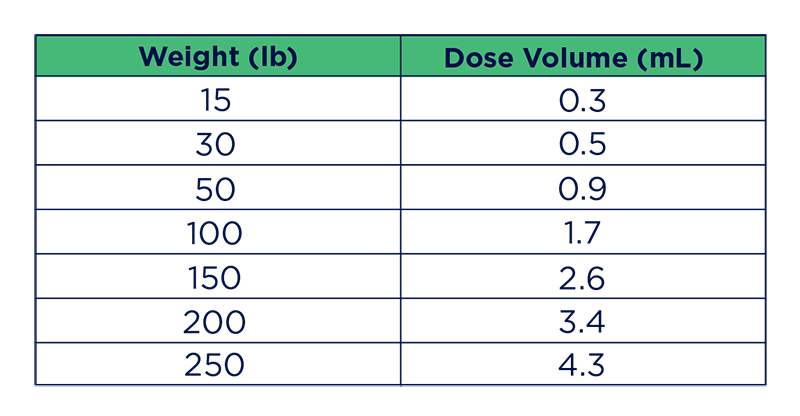
Pradalex Dosing Guide for Swine (1.7 mL/100 lbs)
RESPIRATORY SOLUTIONS
Immune support, preventative measures, and quick interventions against respiratory disease are essential to not only your herd’s health but to the health of your bottom line. Protect your investment with Elanco to address common respiratory disease challenges.
Talk to your Elanco team today about Pradalex product availability and see how Pradalex might help combat respiratory disease in your operation.


Sign up to stay informed about Pradalex and Elanco’s line of respiratory solutions.
The labels contain complete use information, including cautions and warnings. Always read, follow and understand the label and use directions.
PradalexTM
INDICATIONS:
Cattle: Pradalex is indicated for the treatment of BRD associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in cattle intended for slaughter (beef calves 2 months of age and older, growing beef steers, growing beef heifers, and beef bulls intended for slaughter), and in cattle intended for breeding less than 1 year of age (replacement beef and dairy heifers less than 1 year of age and beef and dairy bulls less than 1 year of age). Not for use in cattle intended for breeding 1 year of age and older (replacement beef and dairy heifers 1 year of age and older, beef and dairy bulls 1 year of age and older, and beef and dairy cows), beef calves less than 2 months of age, dairy calves, and veal calves.
Swine: Pradalex is indicated for the treatment of SRD associated with Bordetella bronchiseptica, Glaesserella (Haemophilus) parasuis, Pasteurella multocida, Streptococcus suis and Mycoplasma hyopneumoniae in weaned swine intended for slaughter (nursery, growing, and finishing swine, boars intended for slaughter, barrows, gilts intended for slaughter, and sows intended for slaughter). Not for use in swine intended for breeding (boars intended for breeding, replacement gilts and sows intended for breeding) and in nursing piglets.
DOSAGE AND ADMINISTRATION:
Cattle: Administer once as a subcutaneous injection at a dosage of 10 mg/kg (2.3 mL/100 lb) body weight. Do not inject more than 15 m per subcutaneous injection site.
Swine: Administer once as an intramuscular injection in the neck at a dosage of 7.5 mg/kg (1.7 mL/100 lb) body weight. Do not inject more than 5 mL per intramuscular injection site.
IMPORTANT SAFETY INFORMATION:
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Federal law prohibits the extra-label use of this drug in food-producing animals. To ensure responsible antimicrobial drug use, use of pradofloxacin should be limited to treatment of bovine respiratory disease (BRD) in cattle and treatment of swine respiratory disease (SRD) in swine only after consideration of other non-fluoroquinolone therapeutic options.
